|
Products/Packaged
Systems |
|
|
» |
|
|
» |
|
|
» |
|
|
» |
|
|
» |
|
|
» |
|
|
» |
|
|
» |
|
|
» |
|
|
» |
|
|
» |
|
|
» |
|
|
» |
|
|
» |
|
|
» |
|
|
» |
|
|
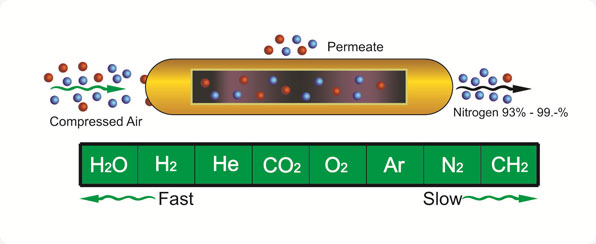
Principle
Membranes separate
gases by the principle of selective permeation across the
membranes wall. For nitrogen polymeric membranes, the rate of
permeation of each gas is determined by its solubility in the
nitrogen membrane material, and the rate of diffusion through the
molecular free volume in the nitrogen membrane wall. Gases that
exhibit high solubility in the nitrogen membranes, and gases that
are small in molecular size, permeate faster than larger, less
soluble gases.
Fast" gases permeate
through the nitrogen membrane wall more readily than "slow" gases,
thus separating the original gas mixture into two streams. The
purity of the desired streams can be adjusted by changing the
operating conditions.
 |
|
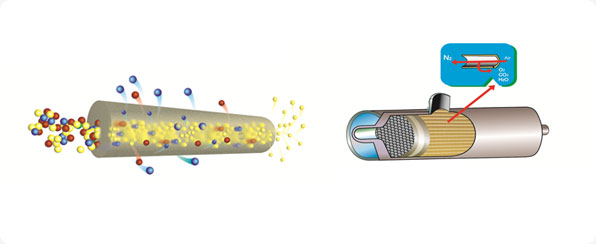
Process
Membrane
processes rely on the fact that under pressure, gases tend
to be permeated through the wall of the hollow fibre
membranes. Faster gases permeate faster than slower gases.
When a treated compressed air passed through the membrane,
water, carbon dioxide and oxygen permeates and vented to
atmosphere where as Nitrogen comes out in the main stream
as the product gas.. It is a continuous process. No
depressurization or regeneration is required |